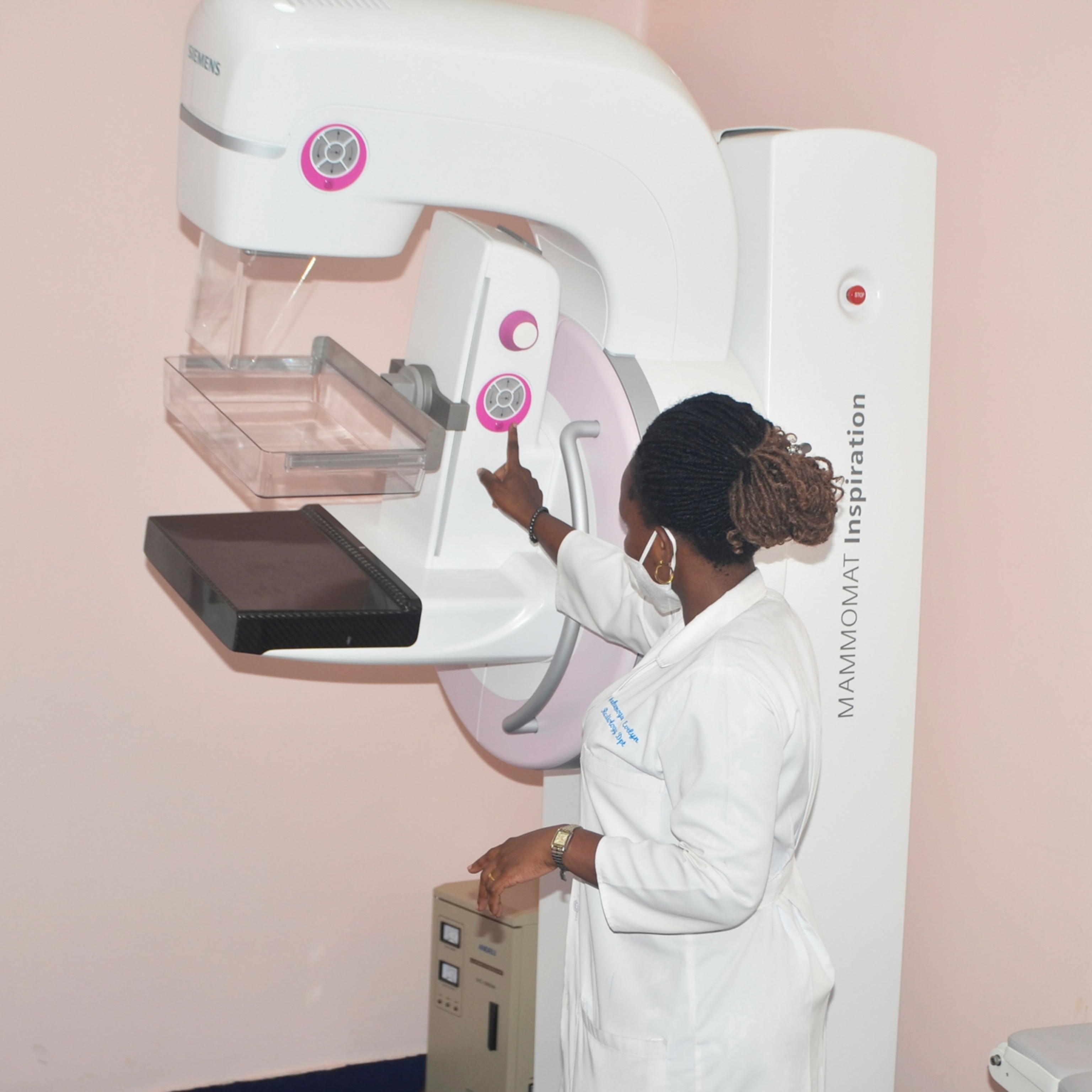
Breast cancer spreads more aggressively during sleep
Many more cancerous cells circulate in the blood at night, suggesting that time of day could play a role in diagnosis and treatment.

More than just a 24-hour biological timer that lulls us asleep at night and awakens in the morning, the inbuilt master clock in the human brain also controls the daily ebb and flow of hormones, regulates body temperature, cues hunger, and schedules digestion, among hundreds of physiological functions. Now a new study of breast cancer patients reveals that cancer cells take advantage of these hormone cycles to spread while the patient is sleeping.
Cancers spread to new locations when cells break away from the original tumor and travel to faraway tissues through blood or lymph systems after escaping across blood vessel walls. This spread or “metastasis” causes most deaths from cancers. Scientists used to think that these free circulating tumor cells, or CTCs, are shed continuously throughout the day into the bloodstream. But a new study shows that in breast cancer patients most CTCs are released during late phase of sleep just before sunrise rather than during the active day hours.
“When the patient is asleep, the tumor awakens,” says Nicola Aceto, a molecular oncologist at the Swiss Federal Institute of Technology (ETH) in Zurich, Switzerland, who led the study.
The new research provides insights into biological mechanisms that allow cancer to spread, and an invaluable observation that physicians can use to track cancer growth and metastasis by taking blood samples on precise schedules.
“We clearly demonstrate that the timing of a biopsy is very critical for better diagnosis,” says Zoi Diamantopoulou, a cancer cell biologist in Aceto’s lab and the study’s lead author.
Scientists made a point of clarifying that the study does not show that cancers are caused by sleeping or rest. What it shows is that once a cancer has established, its progression is affected by the sleep and related hormonal changes. “For patients who already have cancer, consistent sleep is vital to ensuring that the rest of their body is strong enough to endure treatment and fight off the disease,” says Harrison Ball, a graduate student at University of Michigan, who was invited to write a commentary on Aceto’s study.
Sleep promotes a strong immune system which is good for protecting against cancers. “Our immune system is at its best when we get enough rest,” says Sunitha Nagrath, a chemical engineer at University of Michigan Ann Arbor, who develops tools for isolating and studying rare cells from cancer patients. “Cancer might spread aggressively [during the night], but the body can fight back if we have a great immune system.”
The study’s conclusions fill a critical gap in knowledge in cancer biology, “especially finding that CTCs tend to be more numerous and more aggressive at night,” says Francis Lévi, a medical oncologist at France’s Paris-Saclay University, who has spent the last three decades exploring how circadian rhythms influence health and disease. Lévi’s own contributions include showing that the toxicity and side effects of certain cancer drugs could be reduced by altering the time they are administered—a field now known as chronotherapy—and establishing that these times differed for men and women.
“This will still take decades and probably prospective clinical trials to make clinicians realize that time of day has an importance,” says Christoph Scheiermann, an immunologist at University of Geneva in Switzerland. But based on this study, sampling blood at the right times can be of immediate diagnostic importance, Scheiermann believes.
Circadian rhythms of immunity and cancer
Scientists have been studying the day-night biological rhythms, or chronobiology, since the 18th century. French astronomer Jean Jacques d’Ortous de Mairan first showed in 1729 that leaves of the touch-me-not plant continued to open and close during the 24-hour day-night cycle even when plunged into permanent darkness, suggesting that the plant had an inner clock that allowed it to keep track of time. Similar biological clocks tick under cues from the sunlight in all vertebrates, plants, fungi, and bacteria, keeping them in synch with their environment. This explains why some animals, including cats, are nocturnal and some algae brighten their bioluminescence after sunset.
A master clock in the human brain is not an abstract idea. The clock is a collection of approximately 20,000 “clock cell” neurons located in the suprachiasmatic nuclei of the anterior hypothalamus that regulates a 24-hour cycle of physiological and behavioral changes called circadian rhythms (from Latin circa diem, “about a day”). This bundle of neurons receives light signals from the retina and orchestrates the ticking of slave oscillators in other brain regions and organs, including the liver and kidney, by turning on and off a multitude of genes. In 2017 Jeffrey Hall, Michael Rosbash, and Michael Young were awarded the Nobel Prize for the discovery of critical genes that drive the gears of this internal clock. In humans, at least 30 percent of all genes that make proteins show cyclical activity in various organs, a number likely to grow with better chronicling of gene activities.
While a majority of genes in healthy cells are most active during the early morning and late afternoon, others peak in early evening, during sleep when there is no food intake. “Typically, this [synchronization] is done through the release of various signaling molecules, such as hormones, that circulate throughout the body,” Ball says.
In humans, when light levels dip, the circadian clock neurons secrete the sleep hormone melatonin. Genes that produce other hormones—such as hunger-inducing leptin, which coordinates appetite, and stress-responding cortisol, which wakes us up in the morning and fights off disease—also respond to the light and dark cycles.
When human activity conflicts with the 24-hour day-night cycle—such as for shift-workers who are active when it is dark and sleep when it is light—the circadian mismatch increases the risk of developing cancers. Female flight attendants and nurses have modestly increased risk of getting breast cancer, possibly because of disruption in circadian rhythms. In lab studies, mice also show increased risk of developing mammary tumors in simulated shift-work conditions. That is why the International Agency for Research on Cancer considers shift work at odd hours, such as those of flight attendants and night nurses, as “probably carcinogenic.”
The risks of shift work extend beyond breast cancer to prostate cancer, and cardiovascular and several other chronic diseases. This population also has a greater chance of developing infections, says Lévi.
While it isn’t clear why frequent disturbances in sleep-wake cycles are associated with increased risk of cancer, studies suggest that immunosuppression, chronic inflammation, or increased cell proliferation could be the culprit.
“Immune cells also have circadian clock, and their function shows daily fluctuations,” says Kazuhiro Yagita, a doctor of internal medicine at Kyoto Prefectural University of Medicine, Japan.
The levels of circulating white blood cells, which help the body fight infection and other diseases, peak during the rest phase: nights for humans and day for mice. Misalignment between the environmental cycle and the internal circadian clocks causes metabolic havoc in cells leading them to malfunction, Yagita says. On the other hand, “Sleep is very good for protecting or reducing the risk from cancers.”
But once cells become cancerous, they break free of circadian rhythms. “Cancer cells and tumors normally don't exhibit these oscillations that you see in healthy tissues,” says Scheiermann. “They wouldn't know what time it is.”
That is why it comes as a surprise to scientists that key circadian rhythm hormones such as melatonin and testosterone directly affected the dynamics of CTC generation in the Zurich study.
Nocturnal research
When scientists noticed discrepancies in the number of cancer cells detected in blood samples collected at different times from cancer patients, they were motivated to investigate further, Aceto says.
“We found that release of CTCs was not constant during the day, but it would go up and down,” Diamantopoulou says. “It made us excited to keep on looking for how the release of CTCs is regulated.” Scientists suspected that hormones like melatonin, which governs sleep; and corticoids, which balance stress response, energy flow, body temperature, water balance among other essential processes, might be the signals that drive timing of cancer cell shedding. These hormones are known regulators of circadian rhythms and both reach peak levels in the blood between 3 a.m. and 4:30 a.m., so Diamantopoulou collected blood from 30 patients hospitalized with breast cancer, once at 4 a.m. and again at 10 a.m. “I wished it was a bit later or a bit earlier; it would have been more convenient and easier to analyze the samples,” she says.
Diamantopoulou found that almost 80 percent of the CTCs were detected in the blood samples collected at 4 a.m., when the patients were resting. To better understand their results, scientists replicated their findings in mice with experimentally induced cancer. Since mice are nocturnal, their CTC levels went up to 88-fold higher during day when the animals were resting, compared to nights when they were active. Disrupting the sleep-wake cycle of mice decreased the cancer cells in their blood.
During the resting phase, the cancer cells that were released divided more rapidly than healthy cells. These cells were also more likely to grow into new tumors, suggesting that CTCs breaking away during sleep were somehow better at metastasis.
“These circulating tumor cells are probably being shed and then acutely being taken up by another tissue, so for me, that is the most exciting part [of the study],” says Scheiermann.
“It is surprising that not just the number of cells were different, but the rest phase CTCs were more aggressive when compared to CTCs just a few hours later,” says Nagrath.
While melatonin increased the production of CTCs and tumor growth, a chemical compound that blocks melatonin could reverse the effects. Insulin, on the other hand, promoted the proliferation of tumor cells. This suggests that cancer cells still respond to some of the cues from the day-night cycle and circadian rhythm.
“It makes complete sense that circulating cancer cells were more likely to grow and divide while resting—a pattern that is matched by non-cancerous cells as well,” says Ball. This increased proliferation also makes these cells more "aggressive," that is, more likely to spread and form secondary tumors. The results of this study can lead to new ways of taking cancer biopsies, but also address whether treatments should be administered at different times during the day.
While the idea of scheduling the dose of a medicine to match a patient’s circadian rhythm through chronotherapy is still in it’s infancy, Lévi’s research shows that accounting for circadian clock can influence efficacy and tolerance of dozens of cancer medicines. Other trials have hinted that breast, ovarian, and lung cancers could benefit from chronotherapy.
“We found important—up to five-fold—differences in toxicities and near doubling in efficacy of the same chemotherapy regimen, when it was given at chronomodulated infusion compared to a constant rate,” says Lévi. A colorectal cancer trial that Lévi led suggests that scheduling doses matched to circadian rhythms might even have sex-specific benefits for men and women.
Lévi says Aceto’s findings lay an important foundation but there is more to do. “This study only tested two time points, which is not enough,” says Lévi. There can also be variations in circadian rhythms among patients, as seen in the studied mice. “The patients can have different chronotype,” he adds, such as those who are early birds versus those who are night owls. The nature of tumor and the chemotherapy can also disrupt the circadian rhythms of patients.
What’s next? Nagrath says, “One needs to check whether these observations are true for all cancer or only for hormone sensitive cancers [such as breast cancer].”
Related Topics
You May Also Like
Go Further
Animals
- Orangutan seen using plants to heal wound for first timeOrangutan seen using plants to heal wound for first time
- What La Palma's 'lava tubes' tell us about life on other planetsWhat La Palma's 'lava tubes' tell us about life on other planets
- This fungus turns cicadas into zombies who procreate—then dieThis fungus turns cicadas into zombies who procreate—then die
- How can we protect grizzlies from their biggest threat—trains?How can we protect grizzlies from their biggest threat—trains?
Environment
- What La Palma's 'lava tubes' tell us about life on other planetsWhat La Palma's 'lava tubes' tell us about life on other planets
- How fungi form ‘fairy rings’ and inspire superstitionsHow fungi form ‘fairy rings’ and inspire superstitions
- Your favorite foods may not taste the same in the future. Here's why.Your favorite foods may not taste the same in the future. Here's why.
- Are the Great Lakes the key to solving America’s emissions conundrum?Are the Great Lakes the key to solving America’s emissions conundrum?
- The world’s historic sites face climate change. Can Petra lead the way?The world’s historic sites face climate change. Can Petra lead the way?
History & Culture
- Meet the ruthless king who unified the Kingdom of Hawai'iMeet the ruthless king who unified the Kingdom of Hawai'i
- Hawaii's Lei Day is about so much more than flowersHawaii's Lei Day is about so much more than flowers
- When treasure hunters find artifacts, who gets to keep them?When treasure hunters find artifacts, who gets to keep them?
Science
- Why ovaries are so crucial to women’s health and longevityWhy ovaries are so crucial to women’s health and longevity
- Orangutan seen using plants to heal wound for first timeOrangutan seen using plants to heal wound for first time
Travel
- Why this unlikely UK destination should be on your radarWhy this unlikely UK destination should be on your radar
- A slow journey around the islands of southern VietnamA slow journey around the islands of southern Vietnam
- Is it possible to climb Mount Everest responsibly?Is it possible to climb Mount Everest responsibly?
- 5 of Uganda’s most magnificent national parks
- Paid Content
5 of Uganda’s most magnificent national parks